
Veterinary pharmacy in Russia
The Association of Veterinary Pharmaceutical Manufacturers emphasized the relevance of replacing imported drugs after the introduction of the GMP standard. The replacement of high-tech drugs will take a decade, but Russia is already prepared. The restoration of drug supplies is planned by 2025.
The issue of import substitution in veterinary medicine is relevant, especially after the implementation of the GMP standard, said Semen Zhavoronkov, Executive Director of the Association of Veterinary Pharmaceutical Manufacturers (AVFARM), at the industry conference "Agroinvestor: PRO livestock and feed additives."
He emphasized that without quality veterinary drugs, investments in livestock farming are practically impossible. Despite the fact that the cost of pharmaceuticals in the cost of livestock products is only 3%, sometimes even 1-2%, their absence or incorrect use can lead to serious consequences for farms.
Only three global manufacturers provide the majority of veterinary drug supplies. Zhavoronkov noted that it will take a decade to replace high-tech drugs used in agriculture. Even India and China, which started this process earlier than Russia, still import around 30-35% of drugs.
The main focus of import substitution of veterinary drugs is on immunological products, which require multiple stages for creation and registration. Zhavoronkov noted that last year the GMP procedure extended not only to drug registration but also to their introduction into civil circulation. This led to a significant reduction in the assortment of available drugs on the market. However, thanks to large reserves, Russia was able to survive this period.
Special attention in the veterinary drug market is paid to three categories. The first category is vaccines against particularly dangerous diseases, which are fully produced within the country. The second category is vaccines against economically significant diseases, the dependency on imports of which reaches 90-95%. The third category is chemical-pharmaceutical preparations, some of which are partially produced in Russia.
At the moment, 423 foreign vaccines and 1376 drugs are registered in the EAEU, as well as 452 vaccines and 375 drugs from Russia and Belarus. However, due to new requirements for the introduction of drugs into civil circulation, only 12 imported vaccines and 27 foreign chemical drugs are available. At the same time, many domestic vaccines, including those designed to combat particularly dangerous diseases, remain available. Russian companies found it much easier to obtain a GMP certificate compared to foreign ones, many of which were denied certification.
Zhavoronkov warned that even after obtaining certificates, the restoration of drug supplies will take one to one and a half years. He believes that the actual timeline for the restoration of supplies will not be earlier than 2025.
Currently, AVFARM is focused on restoring vaccine supplies for poultry farming, pig farming, and cattle breeding. Regarding other categories of drugs, they are currently maintained in the market, and the necessary volumes are retained in case of obtaining GMP certificates.

 Trading platform
Trading platform 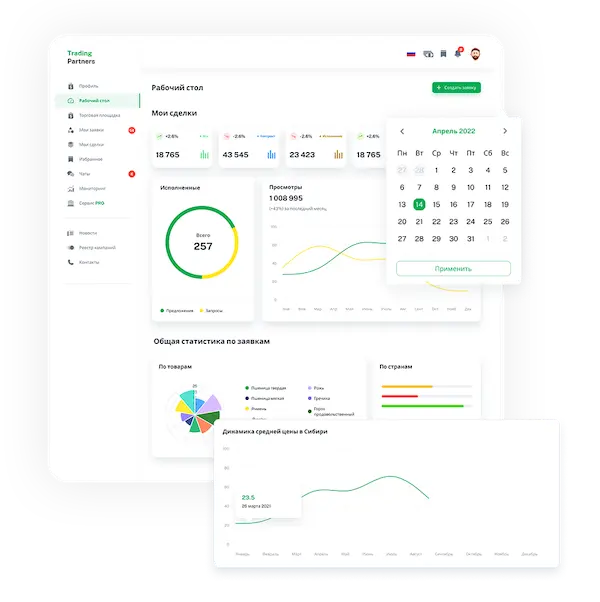
 Monitoring
Monitoring  Express applications
Express applications 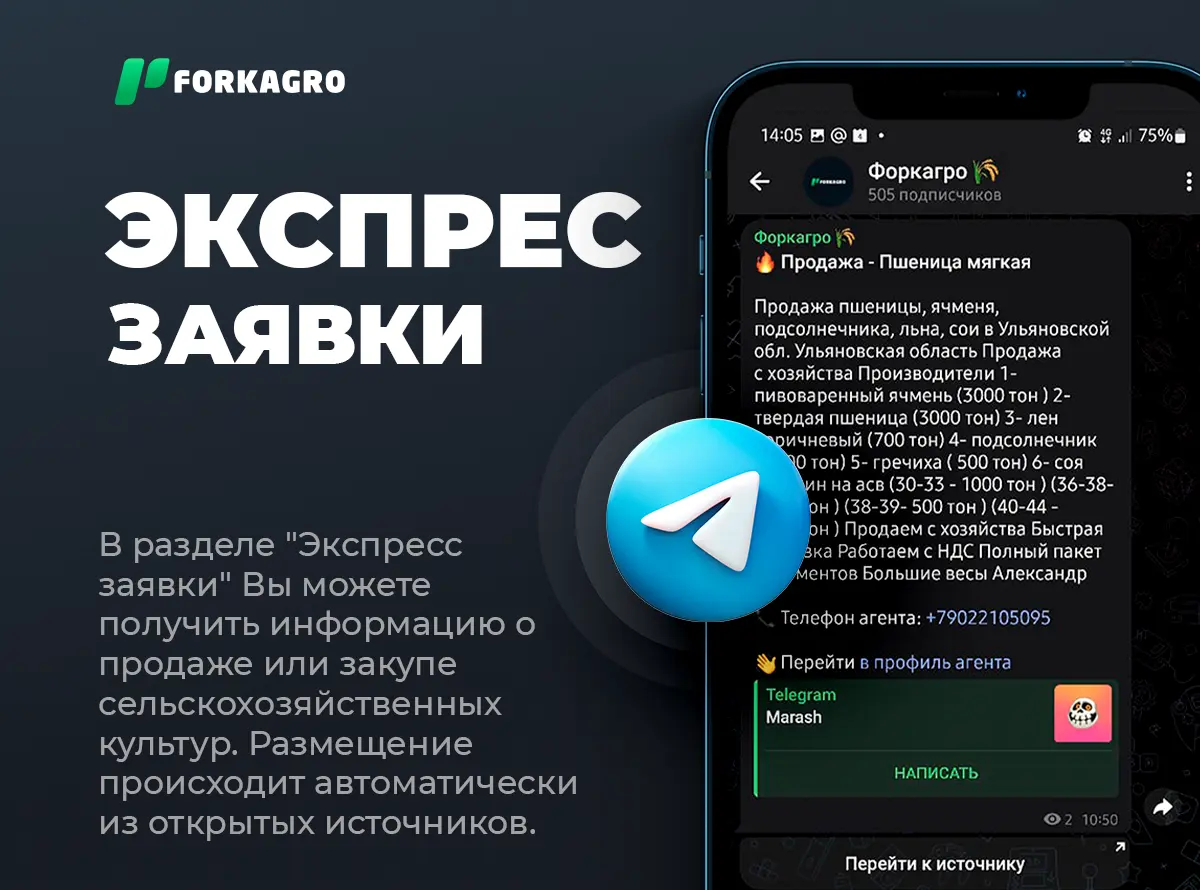
 Fork Work
Fork Work 
 Service
Service  News
News  Directory
Directory 